Guest Bloggers Scott Lidgard & Alan Love write...
At first glance, the notion of a ‘living fossil’ looks straightforward: a lineage survives, relatively unchanged, across deep evolutionary time, and becomes an outlier as evolution transforms other lineages around it. However, we think there are some important conceptual issues lurking here (see Lidgard and Love 2018, Open Access), and others also have worried about living fossils (see, e.g., Derek Turner’s entries: “The Macroevolutionary Puzzle of the Cycads”; “Leave No Trace”; “Plus Ca Change and the Importance of Going Extinct”). To see our particular concern, let’s wind back the clock 160 years to an 1858 letter from Charles Darwin—written on Christmas Eve—that continued a conversation (one of many) with Joseph Hooker about distinctive patterns in the biogeographical distribution of plants and animals.
Charles Darwin (left) and Joseph Hooker (right)
Darwin thought his view that natural selection would be stronger in larger populations inhabiting a greater range could explain some of these living fossil outliers.
“Your facts about distribution are indeed very striking. … I did not for long see the bearing of a conclusion, at which I had arrived, with respect to this subject. It is that species inhabiting a very large area, & therefore existing in large numbers & which have been subjected to the severest competition with many other forms, will have arrived through natural selection, at a higher stage of perfection than the inhabitants of a small area.— Thus I explain the fact of so many anomalous or what may be called “living fossils” inhabiting now only fresh-water, having been beaten out & exterminated in the sea by more improved forms; thus all existing Ganoid fishes [sturgeons, paddlefishes, gars, etc.] are fresh-water as is Lepidosiren [lungfish] & Ornithorhynchus [platypus] &c. ”
Why do outliers like lungfishes and platypus live in a restricted geographical distribution? According to Darwin, they are remnants of previously more wide-spread populations that were squeezed out by better-adapted species. These better-adapted species had lived in larger areas and therefore been under more intense competition. It is an explanation he would include in On the Origin of Species: “These anomalous forms may almost be called living fossils; they have endured to the present day, from having inhabited a confined area, and from having thus been exposed to less severe competition” (Darwin 1859, 107).
For the modern reader, Darwin’s discussion of living fossils is odd. He doesn’t foreground what we typically assume is a critical feature of living fossils: morphological stasis. And yet Darwin did note an example of this type: living representatives of the brachiopod genus Lingula that shared the morphologically simple shells occurring in the oldest fossil-bearing geological layers known at the time. “The Silurian Lingula differs but little from the living species of this genus; whereas most of the other Silurian Molluscs and all the Crustaceans have changed greatly” (Darwin 1859, 313). In the Origin, manuscripts, and letters, Darwin intimated several ways of understanding living fossils, including these two distinct ones (losing remnants of evolutionary battles and lineages exhibiting extreme morphological stasis). His statements have proliferated into many criteria in contemporary evolutionary biology, including:
Prolonged geological duration relative to similar entities
Slow rate of evolutionary change relative to similar entities
Gross similarity to an ancestral fossil
Very low taxonomic richness today compared to the past
Relic geographic range today compared to the past
Phylogenetic inference of specific characters as ancestral
Phylogenetic infernce ofa genealogical divergence between other groups that diverged in the distant past
Known in the fossil record before being discovered alive
This definitional diversity is a natural place to hunt for conceptual issues. We think these different definitions mark something significant, something that encourages a broader rethinking of living fossils and leads us to a more integrated view of the concept (Lidgard and Love 2018).
Whether it is horseshoe crabs, tadpole shrimp, or gingko trees (see below), taxa that allegedly display extraordinary morphological stasis over geological time have called out for a special explanation (Lidgard and Hopkins 2015). Why have these constellations of characters persisted for so long? Yet there is widespread dissatisfaction with the living fossils concept; some have even claimed that it is not scientifically sensible (e.g., Casane and Laurenti 2013, Mathers et al. 2013). Complaints include:
Ill-defined or cross-cutting definitional criteria
Molecular genetic change despite apparent morphological stasis (and vice versa)
Preservational or sampling baises in the fossil record
Misclassification or faulty phylogenetic inference
Confusion about what level of taxonomic hierarchy is in view (e.g., species versus higher taxa, or unrecognized cryptic species)
Lineage originations and evolutionary rates not being reliably derived from fossils or molecular clocks
Problematic expectations that morphological change occurs in concert with biotic and abiotic environmental change
Compare the living (top row) with the long fossilized (bottom row). (see here for species names & sources)
We believe the concept of a living fossil needs to be reanalyzed in light of both its routine use across disparate biological research and worries about potentially misleading inferences, such as presumptions of morphological stability due to preservational bias. Two big ideas are relevant to this rethinking: (1) the value of distinguishing parts and wholes, and (2) the need to move beyond categorizing living fossils. The result is a research program comprised of a diverse array of questions that encompasses different conceptions of living fossils across hierarchical levels of organization (for the full story, see Lidgard and Love 2018).
Parts and Wholes
All scientific practice involves using proxies—measurements of particular properties that stand in for something else. Biologists use subsets of characters (parts) and their differing states to discriminate among organisms or lineages (wholes). For example, in paleontological analyses, hard body parts are vastly more available and abundant than soft body parts. The hard body parts serve as proxies for the lineages studied, frequently in the absence of any preserved soft body parts. However, when biologists are interested in stasis, an ambiguity can emerge between the morphological and molecular parts of an organism and whole organisms or genomes of a lineage or clade. This is because of a common practice: single size and shape characters are taken to represent a species or lineage in most quantitative paleontological studies of evolutionary modes (Hopkins and Lidgard 2012).
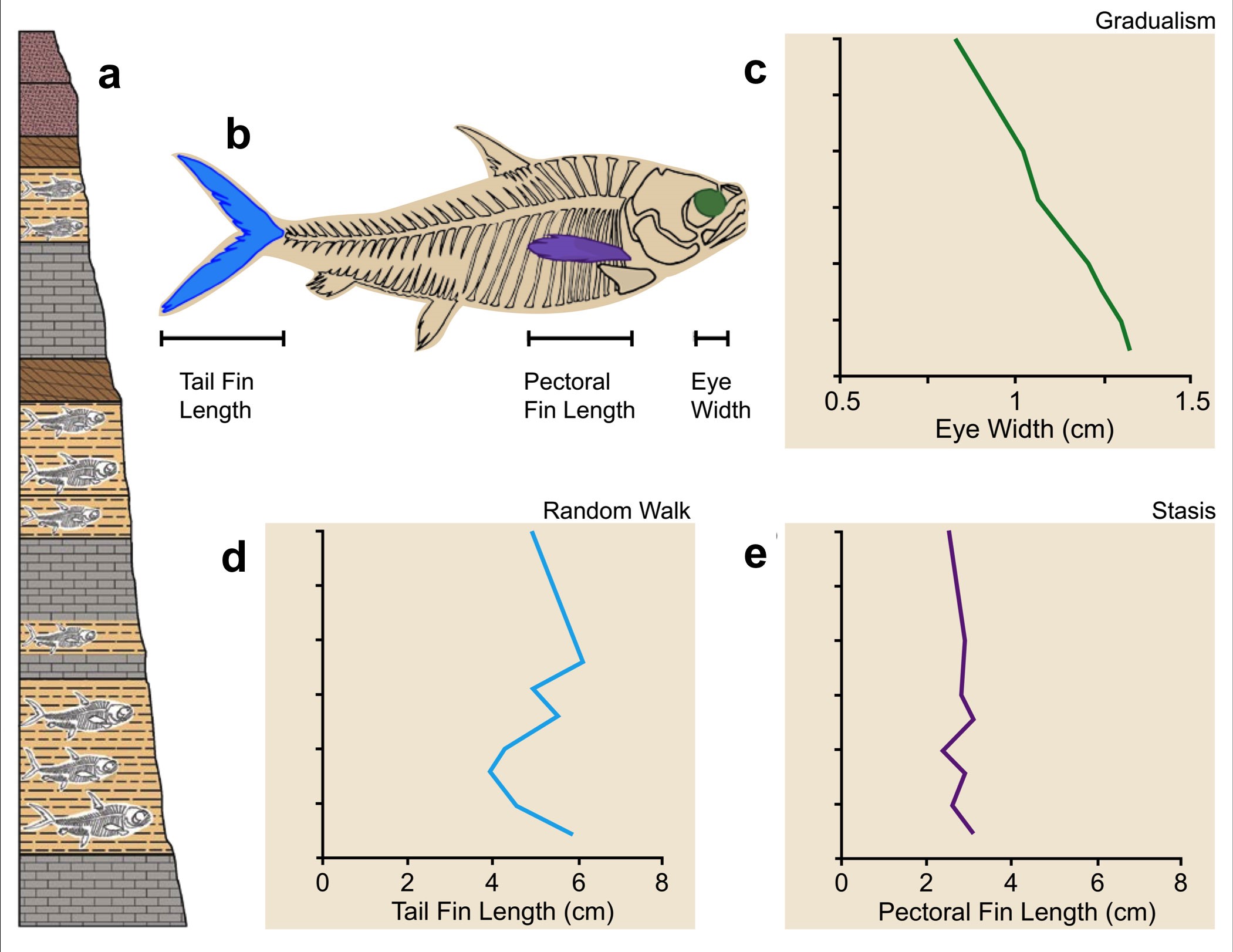
Although this common practice has been fruitful, analyses where multiple characters are recorded for the same species or lineage frequently distinguish different evolutionary modes for different characters (Hopkins and Lidgard 2012, Hunt et al. 2015, Voje et al. 2018). This can be illustrated with a hypothetical lineage of fossil fish (see below). Many characters are available to represent the lineage. Of those available, only a subset is selected to be measured and represent the lineage (in this case, eye width, tail fin length, and pectoral fin length). What turns out to be surprising after doing the quantitative analysis is that each of the three characters is best accounted for by a different model of evolutionary change: gradualism for eye width, random walk for tail fin length, and stasis for pectoral fin length. If we had only used a single size or shape character, it would have generated three very different perspectives on the evolutionary trajectory of the fossil fish lineage. More abstractly, there is a complicated relationship between parts and wholes through evolutionary time. Controversies about categorizing living fossils have not yet reckoned with this complexity.
Ginkgoalean fossils are commonly recognized from their leaves, sometimes indistinguishable from leaves of living Ginkgo biloba (see above). Different developmental stages of modern leaves can be referred to separate fossil species or genera, and different parts that help classify a plant, such as fruit, seeds, or wood, are seldom preserved together as whole plant fossils. The genus may go back ~170 million years, but different morphological part proxies yield separate ages of first appearance (Zhou 2009). In short, living fossil taxa exhibit a mix of characters that have remained stable and those which have changed over evolutionary time. We know that different phylogenetic trees can be reconstructed from different sets of characters, both morphological and molecular, and that these characters exhibit dramatic variation in their rate and amount of evolutionary change (see Adrian Currie’s “The Strange Case of the Crocodile’s Snout. Part 1: Answerless Questions”). When identifying living fossils, what combination of characters sufficiently represents a lineage as a whole?
When we talk of a living fossil “retaining” an ancestral morphology, this retention concerns particular characters or parts. However, if we ask whether the taxon in which these characters appear is geographically widespread, diverse in its extinct representatives, or a low-diversity relict population in the present, then we are tracking wholes—organisms and their lineages. The carapaces of Jurassic Mesolimulus and modern Carcinoscorpius (see above) share numerous characters (parts), yet horseshoe crabs as lineages (wholes) have invaded land multiple times (Lamsdell 2016), with attendant evolutionary changes in ecology, physiology, and morphology. This ambiguity between part and whole goes even further: some taxa labeled as living fossils lack properties we might typically expect, such as geographic relict status, which is not applicable to taxa with extensive geographic ranges like modern horseshoe crabs. These part-whole ambiguities for evaluating evolutionary stasis and change bear directly on controversies about categorizing living fossils.
Beyond Categorization
Criteria for living fossil membership are often criticized as ill-defined or conflicting. This is compounded by the fact that most criteria involve contentious aspects that affect classification judgments (e.g., preservational biases in the fossil record). This nurtures definitional debates about whether particular lineages count as living fossils (e.g., Casane and Laurenti 2013, Mathers et al. 2013), or even whether the concept is justified. Biologists often steer clear of these debates as unproductive (see, for example, conversations about species). One reason for these debates is an assumption that the primary role of a concept is to categorize (i.e., figure out which set of entities should be classified by a particular term); concepts are for distinguishing one thing from another—apples from oranges—or recognizing commonalities—apples and oranges are both fruit. However, concepts play many roles in scientific reasoning (and elsewhere), and this provides an avenue out of definitional debates about what is (or is not) a living fossil.
We can move beyond questions of classification by noting that scientific concepts also play a key role in representing broad investigative domains (Brigandt and Love 2012). The living fossil concept marks out what requires explanation in a given instance of morphological or molecular stability over long periods of evolutionary time. This shift moves us away from semantics and toward both productive research and a more unified conceptual framework for living fossils in three ways.
First, different conceptions and criteria of living fossils derive from different explanatory expectations, whether it is the unspecialized morphology of Lingula, taxonomically intermediate status of the duck-like platypus, or only having remnants of a broad ancient distribution (e.g., Gingko). The multiple conceptions of living fossils represent distinct explanatory questions that require different operational definitions to gather the data required to test hypotheses. In these examples, criteria of adequacy diverge and range from when or where observations are made to what results are derived from a phylogenetic analysis.
Second, increased sampling of the fossil record changes how different conceptions apply to perceived rates and status. Recent work on Crocodyliformes—classic living fossils—has uncovered diverse ecological adaptations, shifting evolutionary rates, and tangents from the generalized crocodylian morphology (Bronzati et al. 2015; pictured below, Simosuchus). Discoveries may also affect fossil age calibrations, changing estimates of taxon ages, divergences, and rates in morphological and molecular phylogenetic results (Wagner and Marcot 2013). More and better-understood fossils in morphological phylogenetic analyses also can change relationships among stem groups and consequent molecular clock calibrations.
Finally, molecular methods have introduced new ‘parts’ of lineages to evaluate for evolutionary stasis. Molecular-oriented researchers pursue different part proxies than do morphological researchers. That genetically identified cryptic species are more common than once thought puts pressure on past assumptions of lineage stasis. More generally, gene sequences—as molecular proxies—show patterns that are often discordant with morphological evidence of living fossil status. Rapid rates of change in some molecular characters have prompted a reevaluation of classic living fossils (Casane and Laurenti 2013).
A Biological Research Program
Two further items help us to understand how the living fossil concept plays the role of marking out what requires explanation and structures a research program around different factors associated with long-term stasis: (a) shared criteria of adequacy and (b) relationships among research questions of different kinds.
(a) Shared criteria of adequacy are evaluative standards for deciding whether descriptions or explanations meet the aims of a research community. Living fossil and stasis are relative terms, established by comparison: what’s changing (or not)? How fast? In relation to what? By what assumptions or theoretical model? Making the metric(s) of comparison overt and interpretable helps to define a domain within which specific questions can be asked (and answered).
(b) Thematic and dependency relationships between different types of questions include: How many characters count as a constellation? How is “negligible rate of evolutionary change” operationalized? What do “geographically widespread”, “diverse”, “relict”, and the “same” lineage or clade mean? Different criteria need to be made explicit in different contexts and in relation to other allied questions to explain slow or negligible rates of evolutionary change for constellations of molecular or morphological characters in genealogical lineages.
Overall, the living fossil concept can be understood as setting an integrated agenda for research—interrelated suites of questions about patterns in need of explanation and processes relevant to specific character constellations and wholes—that advances our understanding of evolutionary stasis across hierarchical levels of organization. Relationships among questions provide a means to navigate the complex architecture of the living fossils problem agenda:
- What mechanisms are responsible for the retention of particular groups of ancestral morphological characters over long periods of time within a lineage?
- For slow rates of change, how are suites of morphological and molecular characters related?
- Why do some but not all constellations of characters exhibit apparent stasis over long periods of time in the same lineage?
- Why do constellations of characters that represent defining features of species persist over long durations and exhibit little net evolutionary change when compared to other lineages?
- How are perceived declines in living fossil groups (from previous high levels of taxonomic diversity to low levels today) related to patterns in phylogenetic sister groups, and to origination and extinction dynamics?
- Why do some living fossils exhibit ‘relict’ geographic distribution (i.e., distribution that is significantly more restricted than in the geologic past)?
- How is molecular or morphological stasis for constellations of characters affected by their manifestation at different junctures in a life history?
- What are our null expectations concerning living fossils?
All of these research questions are interrelated, and therefore better characterizations of different patterns of stasis and accounts of the mechanisms underlying them fosters conceptual unification for diverse investigations of living fossils.
Conclusion
The research questions detailed above correspond to several of the cross-cutting membership criteria that make the living fossil concept contentious. Thinking explicitly about relationships between parts and wholes requires revisions in how we understand stereotypical living fossils. Our argument suggests a parallel interpretation of highly conserved molecules and genetic mechanisms: the relative stability of bundles of molecular characters represents an ongoing explanatory task for contemporary biologists. Instead of viewing the living fossil concept in terms of categorization—what criteria should define living fossils—it is better to understand its role as setting an integrated agenda for research. Our synthesis (Lidgard and Love 2018) consolidates a broad array of heterogeneous and fragmented investigations, provides a more unified framework that steers clear of semantic debates, and facilitates interdisciplinary research on evolutionary questions surrounding molecular and morphological stasis. The main point can be distilled into a pithy question and reply:
Question: is coelacanth a living fossil?
Answer: it depends. Why are you asking?
References
Brigandt I, Love AC. 2012. Conceptualizing evolutionary novelty: moving beyond definitional debates. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 318: 417–427.
Bronzati M, Montefeltro FC, Langer MC. 2015. Diversification events and the effects of mass extinctions on Crocodyliformes evolutionary history. Royal Society Open Science 2: 140385.
Casane D, Laurenti P. 2013. Why coelacanths are not ‘living fossils.’ BioEssays 35: 332–338.
Hopkins MJ, Lidgard S. 2012. Evolutionary mode routinely varies among morphological traits within fossil species lineages. Proceedings of the National Academy of Sciences USA 109: 20520–20525.
Hunt G, Hopkins MJ, Lidgard S. 2015. Simple versus complex models of trait evolution and stasis as a response to environmental change. Proceedings of the National Academy of Sciences USA 112: 4885–4890.
Lamsdell JC. 2016. Horseshoe crab phylogeny and independent colonizations of fresh water: ecological invasion as a driver for morphological innovation. Palaeontology 59: 181–194.
Lidgard S, Hopkins M. 2015. Stasis. Oxford Bibliographies in Evolutionary Biology 1–31: www.oxfordbibliographies.com/view/document/obo-9780199941728/obo-9780199941728-0067.xml
Lidgard A, Love AC. 2018. Rethinking living fossils. Bioscience. doi:10.1093/biosci/biy084 (Open Access)
Mathers TC, Hammond RL, Jenner RA, Hänfling B, Gómez A. 2013. Multiple global radiations in tadpole shrimps challenge the concept of ‘living fossils.’ PeerJ 1: e62.
Voje, Kjetil Lysne, Jostein Starrfelt, and Lee Hsiang Liow. 2018. “Model Adequacy and Microevolutionary Explanations for Stasis in the Fossil Record.” The American Naturalist 191 (4): 509–523.
Wagner PJ, Marcot JD. 2013. Modelling distributions of fossil sampling rates over time, space and taxa: assessment and implications for macroevolutionary studies. Methods in Ecology and Evolution 4: 703–713.
Zhou, Z. 2009. An overview of fossil Ginkgoales.” Palaeoworld 18: 1–22.